EU Facility - Waterford, Ireland
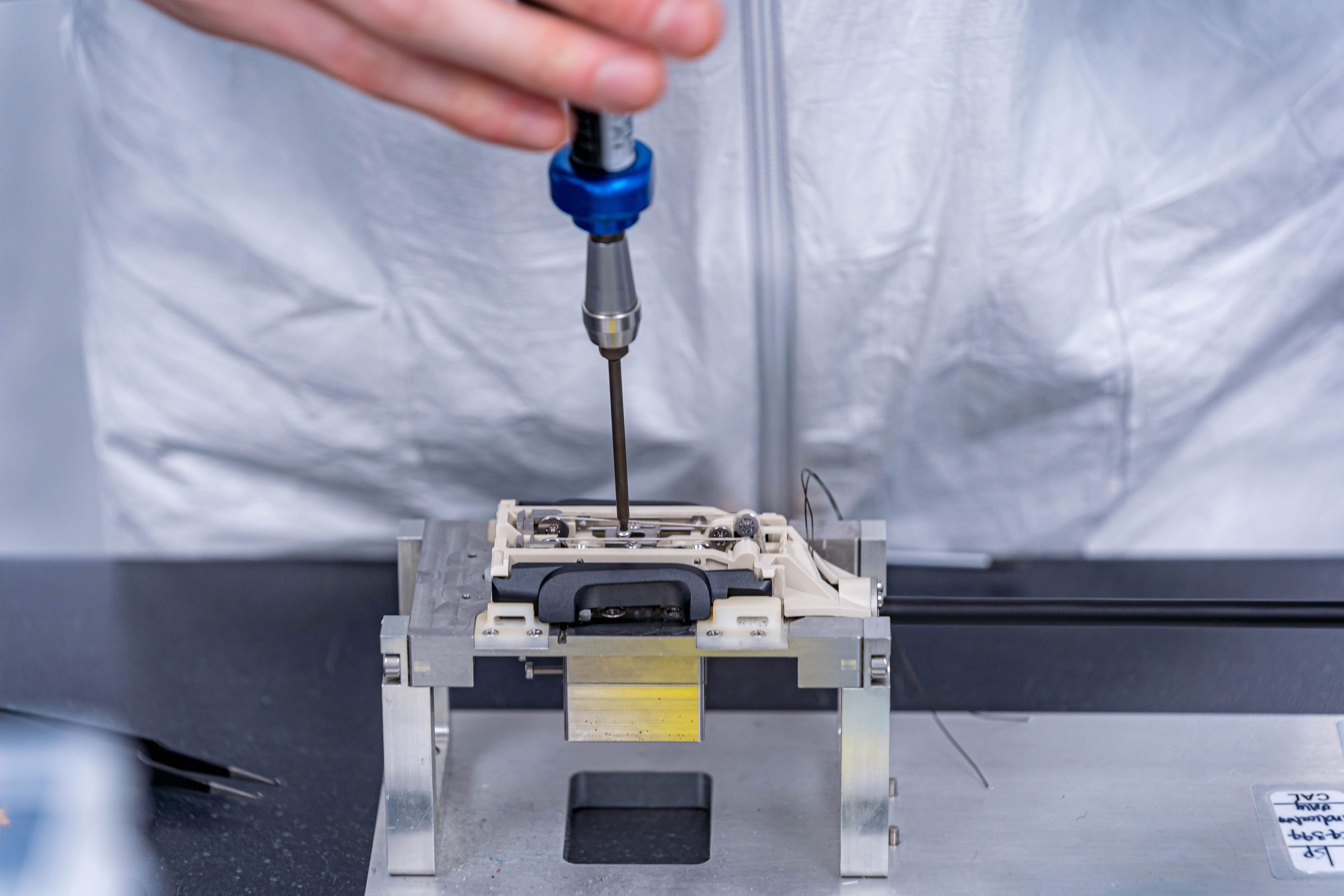
Here you may view our flagship facility in Waterford, Ireland - a 95,000 sq ft campus with state-of-the-art Class 10,000 & 100,000 (ISO classes 7 & 8) electro-mechanical manufacturing cleanrooms, CNC Machining, laser technology, and more. Our dedicated on-site staff perform a full suite of services, including, but not limited to, component manufacturing, sub-assembly and full assembly, testing, inspection, packaging, labeling, sterilization, and more.Precision Engineering





Complex Manufacturing




Simplified Supply Chain




Assembly and Cleanrooms







Our History and Values




